Abstract
Background: Copy-neutral loss-of-heterozygosity (CN-LOH) - not detectable by chromosome banding analysis - is gaining importance as a prognostic factor and can either cause the duplication of an activating mutation in an oncogene, the deletion of a tumor suppressor gene or the gain/loss of specific methylated regions. However, examination for possible CN-LOH in hematological diagnostics is at present not routinely performed and, hence, data regarding the occurrence of CN-LOH across different entities as well as the association of relevant genes is limited.
Aim: (1) Frequency assessment of CN-LOH by target enrichment sequencing (TES) in a diagnostic setting, (2) evaluation of whole genome sequencing (WGS) data to estimate the prevalence of CN-LOH in a larger cohort, to pinpoint relevant genes for CN-LOHs with so far unknown associations, and to determine cross-entity variability.
Patients and Methods: 1196 patients (507 female, 689 male, median age: 66 years), sent between 04/2021-07/2021 for diagnostic work-up, were analyzed by TES with a median coverage of 1765x for the gene panel and 52x for the CNV spike-in panel (IDT, Coralville, IA). Amplification-free WGS libraries of 3851 different patients were sequenced with a median coverage of 102x. Reads were aligned to the human reference genome (GRCh37, Ensembl annotation, Isaac aligner). Cnvkit (v 0.9.9) was used to call copy number variations (CNVs) and CN-LOH for TES and HadoopCNV (Yang et al. 2017) was used to call CN-LOH for WGS.
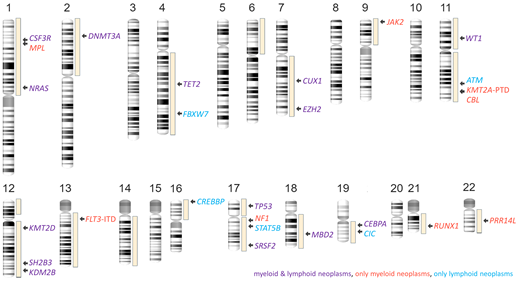
Results: 1196 patients were analyzed by TES. For 10% of the patients at least one CN-LOH event was detected without any association to age or gender but a slightly higher incidence in myeloid compared to lymphoid neoplasms (10% vs 6%). In 14 patients, CN-LOH affected more than one chromosome arm. CN-LOH occurred most frequently in 4q (n = 15), 7q (n = 16), 9p (n = 25) and 11q (n = 10). As expected, 4q CN-LOH co-occurred with high variant allele frequencies (VAF) of TET2. Based on WGS data, 4q CN-LOH occurred predominately in AML (35%), CMML (22%), and MDS (20%). In rare cases, 4q CN-LOH was associated with FBXW7 variants in T-ALL. 7q CN-LOH occurred nearly exclusively in myeloid neoplasms (95%) and was associated with high VAFs in EZH2 in 69% of TES and 82% of WGS cases. CUX1 variants with high VAFs were detected in 80% (TES) and 45% (WGS) of the remaining cases, respectively. The well-known 9p CN-LOH led to JAK2V617F homozygosity in all myeloid neoplasms and occurred most often in MPNs. In T-ALL, regions of 9p CN-LOH harbored CDKN2A/B deletions. 11q CN-LOH occurred more often in myeloid than lymphoid neoplasms (79% vs 21%) and was associated with CBL variants in 61% and KMT2A-PTD in 19% of the cases. In contrast, ATM was the relevant gene in all lymphoid cases with 11q CN-LOH. CN-LOH in 11p was detected less frequently and only in 25% of cases an association with WT1 variants could be identified.
Our WGS data confirmed the known associations between 1p CN-LOH and high allele burden in MPL, CSF3R and NRAS, 2p CN-LOH and DNMT3A variants, 13q CN-LOH and FLT3-ITD, the near exclusive occurrence of 16p CN-LOH in follicular lymphoma (FL, 98%) with high CREBBP-mutant allele burden , 17p CN-LOH and TP53 homozygosity, and the exclusive occurrence of 21q CN-LOH in AML and its association with RUNX1 mutations. Besides, 12q CN-LOH was associated with KMT2D in FL, with SH2B3 in MDS/MPN overlaps and in rare cases with KDM2B. For 17q CN-LOH the relevant gene was not unequivocally identifiable with high mutant allele variants in SRSF2, STAT5B, and NF1. 18q CN-LOH was a very rare event but consistently associated with a high VAF of MBD2, which presumably influences cell proliferation (Cheng et al. 2018). 19q CN-LOH was mostly (63%) associated with a high VAF of CEBPA variants, except for patients with hairy cell leukemia: in these cases nonsense mutations in CIC (VAF > 90%) were detected. CN-LOH in 22q was more common in myeloid malignancies (65% vs 35%) and associated with PRR14L mutations in the majority of myeloid cases (62%). Of note, this association occurred neither in AML samples nor in lymphoid neoplasms. No recurrent mutations were found for 6p and 14q CN-LOHs. For all other chromosomes, CN-LOH events were very rare.
Conclusions: By using a CNV spike-in panel, TES adds additional diagnostic and prognostic information by enabling simultaneous detection of selected gene mutations and genome-wide CNVs, as well as CN-LOH, without increase in sequencing costs and turn-around times.
Haferlach: MLL Munich Leukemia Laboratory: Other: Part ownership. Kern: MLL Munich Leukemia Laboratory: Other: Part ownership. Haferlach: MLL Munich Leukemia Laboratory: Other: Part ownership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal